|
Cleaning Strategy / Sterilization / Autoclave / Glassware
Washer
Sterilization (microbiology)
https://en.wikipedia.org/wiki/Sterilization_(microbiology)
Sterilization (or sterilisation),
referring to any process that eliminates, removes, kills, or deactivates all
forms of life and other biological agents (such as fungi, bacteria, viruses,
spore forms, prions, unicellular eukaryotic organisms such as Plasmodium,
etc.) present in a specified region, such as a surface, a volume of fluid,
medication, or in a compound such as biological culture media.[1][2] Sterilization can be achieved through
various means, including: heat, chemicals, irradiation, high
pressure,
and filtration.
Sterilization is distinct from disinfection,
sanitization, and pasteurization in that sterilization kills,
deactivates, or eliminates all forms of life and other biological agents which
are present.
|

Front-loading autoclave |
Applications:
Foods:
|

|
One of the first steps toward sterilization was made by Nicolas Appert who discovered that thorough
application of heat over a suitable period slowed the decay of foods and various
liquids, preserving them for safe consumption for a longer time than was
typical. Canning of foods is an extension of the same
principle, and has helped to reduce food borne illness ("food poisoning"). Other methods of
sterilizing foods include food
irradiation[3] and high pressure
(pascalization).[4] |
|
Medicine and surgery:
In general, surgical instruments and medications that enter an already aseptic
part of the body (such as the bloodstream, or penetrating the skin) must be
sterile. Examples of such instruments include scalpels, hypodermic needles and artificial pacemakers.
This is also essential in the manufacture of parenteral pharmaceuticals.
Preparation of injectable medications and intravenous solutions for fluid replacement therapy requires not only sterility
but also well-designed containers to prevent entry of adventitious agents after initial product sterilization.
Most medical and surgical devices used in healthcare
facilities are made of materials that are able to go under steam sterilization.
However, since 1950, there has been an increase in medical devices and
instruments made of materials (e.g., plastics) that require low-temperature
sterilization. Ethylene oxide gas has been used since the 1950s for heat- and
moisture-sensitive medical devices. Within the past 15 years, a number of new,
low-temperature sterilization systems (e.g., hydrogen peroxide gas plasma,
peracetic acid immersion, ozone) have been developed and are being used to
sterilize medical devices.][5]
|

Joseph
Lister was a pioneer of antiseptic surgery. |
|
Steam sterilization is the most widely used
and the most dependable. Steam sterilization is
nontoxic, inexpensive, rapidly microbicidal, sporicidal, and rapidly heats and
penetrates fabrics.[6] |
Quantification
|
|
|
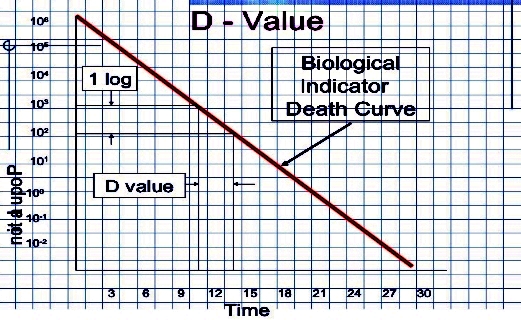
The aim of sterilization is the
reduction of initially present microorganisms or other potential pathogens. The
degree of sterilization is commonly expressed by multiples of the decimal
reduction time, or D-value, denoting the time needed to reduce
the initial number  to one tenth ( to one tenth ( ) of its original value.[8] Then the number of microorganisms ) of its original value.[8] Then the number of microorganisms  after sterilization time after sterilization time  is given by: is given by:
 . .
The D-value is a function of sterilization conditions and varies with the type
of microorganism, temperature, water activity, pH etc.. For steam sterilization (see
below) typically the temperature (in °Celsius) is given as index.
|
|
|
|
Theoretically, the likelihood of survival of an individual microorganism is
never zero. To compensate for this, the overkill method is often used. Using the
overkill method, sterilization is performed by sterilizing for longer than is
required to kill the bioburden present
on or in the item being sterilized. This provides a sterility
assurance level (SAL) equal to the probability
of a non-sterile unit. |
|
For high-risk applications such as medical devices and
injections, a sterility assurance level of at least 10−6 is required by the United
States Food and Drug
Administration (FDA).[9] |
Steam(Moist heat sterilization)
https://en.wikipedia.org/wiki/Sterilization_(microbiology)#Heat
https://en.wikipedia.org/wiki/Moist_heat_sterilization |
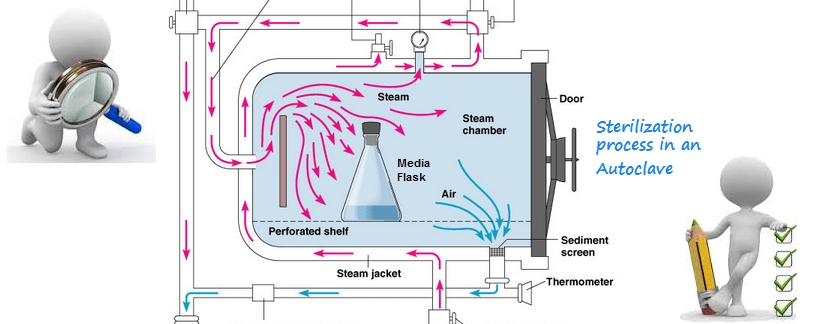
A widely used method for heat sterilization is the Autoclave, sometimes called a
converter or steam sterilizer.
Autoclave :
https://en.wikipedia.org/wiki/Autoclave
An autoclave is a pressure chamber used to carry
out industrial processes requiring elevated temperature and pressure different
from ambient air pressure.
Autoclaves are used in medical applications to perform sterilization and in the
chemical industry to cure coatings and vulcanize rubber and for hydrothermal
synthesis. They are also used in industrial applications, especially
regarding composites, see autoclave
(industrial). |
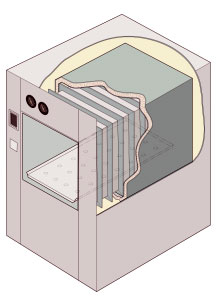
Cutaway illustration of a jacketed rectangular-chamber autoclave |
Action on micro-organisms
Moist heat causes destruction of micro-organisms by denaturation of macromolecules, primarily proteins.
Destruction of cells by lysis may also play a role. While "sterility" implies
the destruction of free-living organisms which may grow within a sample,
sterilization does not necessarily entail destruction of infectious matter. Prions are an example of an infectious agent
that can survive sterilization by moist heat, depending on conditions. |
Many autoclaves are used to sterilize equipment and supplies by subjecting
them to high-pressure saturated steam at 121 °C (249 °F) for around 15–20
minutes depending on the size of the load and the contents.[1]
The autoclave was invented by Charles Chamberland in 1879,[2] although a precursor known as the steam digester was created by Denis Papin in 1679.[3] The name comes from Greek auto-, ultimately meaning self,
and Latin clavis meaning key, thus a self-locking
device.[4] |

|
|
|
Air removal:
It is very important to ensure
that all of the trapped air is removed from the autoclave before activation, as
trapped air is a very poor medium for achieving sterility.
Steam at 134 °C can achieve in three minutes the same sterility that hot air at
160 °C can take two hours to achieve.[8] Methods of air removal include:
Downward displacement (or gravity-type):
As steam enters the chamber, it fills the upper areas first as it is less dense
than air. This process compresses the air to the bottom, forcing it out through
a drain which often contains a temperature sensor. Only when air evacuation is
complete does the discharge stop. Flow is usually controlled by a steam
trap or a solenoid
valve, but bleed holes are sometimes used,
often in conjunction with a solenoid valve. As the steam and air mix, it is also
possible to force out the mixture from locations in the chamber other than the
bottom.
Steam pulsing:
air dilution by using a series of steam pulses, in which the chamber is
alternately pressurized and then depressurized to near atmospheric pressure
Vacuum pumps:
vacuum pump sucks air or air/steam mixtures
from the chamber. (Explained here).
|
Superatmospheric cycles:
achieved with a vacuum pump. It starts with a vacuum followed by a steam pulse
followed by a vacuum followed by a steam pulse. The number of pulses depends on
the particular autoclave and cycle chosen.
Subatmospheric cycles:
similar to the superatmospheric cycles, but chamber pressure never exceeds
atmospheric pressure until they pressurize up to the sterilizing temperature. |
|
|
|
Uses : In medicine
|
A medical autoclave is a device that uses steam to sterilize equipment and other objects. This means that all bacteria, viruses, fungi,
and spores are inactivated. However, prions,
such as those associated with Creutzfeldt–Jakob
disease,
may not be destroyed by autoclaving at the typical 134 °C for three minutes or
121 °C for 15 minutes.[citation
needed] Although a wide range species of archaea, including Geogemma barosii,
can survive at temperatures above 121 °C, no archaea are
known to be infectious or pose a health risk to humans; in fact their
biochemistry is so vastly different from our own and their multiplication rate
is far too slow for microbiologists to worry about them. |
|
|
Autoclaves are found in many medical settings,
laboratories, and other places that need to ensure the sterility of an object.
Many procedures today employ single-use items rather than sterilizable, reusable
items. This first happened with hypodermic needles,
but today many surgical instruments (such as forceps, needle holders, and scalpel handles) are commonly single-use
rather than reusable items (see waste autoclave).
Autoclaves are of particular importance in poorer countries due to the much
greater amount of equipment that is re-used. Providing stove-top or solar
autoclaves to rural medical centers has been the subject of several proposed
medical aid missions.[citation
needed]
|
|
Because damp heat is used, heat-labile products (such as some plastics) cannot be
sterilized this way or they will melt. Paper and other products that may be damaged
by steam must also be sterilized another way. In all autoclaves, items should
always be separated to allow the steam to penetrate the load evenly. |

|
Bio-Hazards:
Autoclaving is often used to sterilize medical waste prior to disposal in the
standard municipal solid
waste stream. This application
has become more common as an alternative to incineration due to environmental and health
concerns raised because of the combustion by-products emitted by incinerators,
especially from the small units which were commonly operated at individual
hospitals. Incineration or a similar thermal oxidation process is still
generally mandated for pathological waste and other very toxic and/or infectious
medical waste. |
|
In dentistry, autoclaves provide sterilization of dental
instruments according to health technical memorandum 01-05 (HTM01-05). According
to HTM01-05, instruments can be kept, once sterilized using a vacuum autoclave
for up to 12 months using sealed pouches.[9] |
Validations |
|
To
facilitate efficient sterilization by steam and pressure, there are several
methods of verification and indication used; these include color-changing
indicator tapes and biological indicators. When using biological indicators,
samples containing spores of heat-resistant microbes such as Geobacillus
stearothermophilis are
sterilized alongside a standard load, and are then incubated in sterile media
(often contained within the sample in a glass ampule to be broken after
sterilization). A color change in the media (indicating acid production by
bacteria; requires the medium to be formulated for this purpose), or the
appearance of turbidity (cloudiness
indicating light scattering by bacterial cells) indicates that sterilization was
not achieved and the sterilization cycle may need revision or improvement. |
Directives / Standards / Certifications
CE PED: Pressure Equipment Directive
https://en.wikipedia.org/wiki/Pressure_Equipment_Directive
EN ISO 9001: Quality management
systems standards
https://en.wikipedia.org/wiki/ISO_9000
BS OHSAS
18001:Occupational Health and Safety Assessment Series
https://en.wikipedia.org/wiki/OHSAS_18001
ASME: American
Society of Mechanical Engineers
https://en.wikipedia.org/wiki/ASME
UNI EN ISO 14001:
Environmental audit
https://en.wikipedia.org/wiki/Environmental_audit
EN ISO 13485 : Medical devices --
Quality management systems
https://en.wikipedia.org/wiki/ISO_13485
EN 285: Sterilization - Steam
sterilizers - Large sterilizers
http://www.din.de/en/meta/search/61764!search?query=en285
DIN 58951-2: Sterilization - Steam
sterilizers for laboratory use - Part 2: Apparatus requirements, requirements on
services and installation
http://www.din.de/en/meta/search/61764!search?query=DIN+58951-2
VDI 6300 Blatt 1:
Genetic engineering operations in genetic engineering facilities - Guidance on
safe operation of genetic engineering facilities
http://www.din.de/en/wdc-beuth:din21:249728861
HTM 2010: Health technical
memorandum 2010 :Part 3: Validation and Varification - Sterilization
https://www.gov.uk/government/collections/health-technical-memorandum-disinfection-and-sterilization
TRB402/DIN EN 61010-2-040 : Thermolock for the sterilization of liquids Safety
requirements
http://www.din.de/en/ |
Applications
|
Bio-pharmaceutical |
Packaging |
Food |
Cosmetics |
Microbiology |

|

|

|

|

|
Loads:
Machine parts, Large volume parenterals,
Syringes, Blood bags, Glassware, Sealed & unseald containers, Garments, Wrapped
tools, Culture media, Tools, Canned food.
User groups:
Microbiology and analytical labs
Research institutes, examination agencies, universities and high schools
Bio-Technologies and life sciences institutions
Clinical diagnostic labs, Hospital operating theaters and medical care, clinical
diagnostic labs
Agriculture, environmental and veterinary labs, Animal facility
Material testing laboratories
Quality control labs in pharma, food/beverage, chemistry/cosmetics and other industrial
sectors.
High Performance Autoclaves Key features:

|
Standard & Certifications:
cGLP compliance, compatible data management.
Comply BSL3–BSL4 laboratory risk category and for operating theater.
Satisfies the most rigid requirements of safety and quality according to
international standards.
The operating software is fully validated and documented.
Optimized loading for Waste with high pathogen risk, and sealed containers.
Single or double doors for Pass-Through regulation and applications.
Engineered and pre-validated according to GAMP5.
Fully validated and documented.
Experience in the pharmaceutical industry across 60 years of history.
System controls:
Industrial microprocessor, graphic LCD, key pad.
Close loop feedback PID algorithm, significant energy saving.
Continuous and accurate control of chamber temperature and pressure.
Industrial lab process controller, high process reliability.
Exclusive pressure and temperature dual sensor system, double regulation and
control of the sterilization process.
High level, fully programmability and control versatility, 30 cycles easy to
customize in a multi-user environment.
Sterilizing Chambers &
Lids:
Volume ranges: 30L, 45, 50, 75, 140, 147, 210, 325, 456,
481, 590, 615, 700, 730 Liters.
High tech chamber and door construction.
Top AISI 316L Stainless Steel, electro, mirror polished sanitary finishing.
AISI 304 Stainless Steel steam recovery tank.
Temperature ranges and High-pressure: 3,5bar, 100 °C-144 °C, customizable.
Internal 316Lss heat-exchanger plates for chamber pre-heating by steam, and cool
down by cold softened water.
Optionally these plates can be used for drying purposes at the end of the cycle.
Energy and water cost-saving. Small foot print with superior loading capacity.
Chamber Lids:
Automatic independent operation and control.
Patented pneumatic lid sealing, assuring maximum safety.
Dedicate buffer air compressor for lid closing and final drying.
Lid with quick closing and safety interlocks.
Exclusive patented swiveling pneumatic gasket"rotate-and-seal"for horizontal
lid.
Guarantees perfect airtight, maximum reliability.
Improved safety and easy maintenance.
Can be equipped with safety device in compliance with TRB402/DIN EN 61010-2-040
and thermal interlock to prevent door open Internal heat exchanger for lid rapid
cooling.
Pneumatically activated Sliding doors for space efficiency.
Single/double Stailess Steel sealing flange (BIOSEAL)
Steam generator:
Built-in with aut- water feed pump.
Heat recovering system for water preheating.
Assuring utilities cost saving.
Steam heat recovery exchanger external to chamber for preheating and reusing
water.
Filetrs:
Vertical position, avoiding frequent rupture.
Components:
Sanitary pneumatic diaphragm valve.
AISI 316L stainless steel components.
Electropolished mirror finished chamber.
AISI 316Lss internal heat exchanger for Chamber Lid.
Chemical inert insulation, both thermal and acoustic smoother, quieter and safer
operations.
User flexibility and interfaces:
Quick connections to all utilities for easy handling and
installation.
Ergonomically designed chamber for maximizing loadable capacity.
Ergonomic features for easy handling and daily use.
Ergonomic positioning of adjustable operator display.
Ideal positioning of operator's panel and printer.
Optimized loading height from floor.
Timed free steaming with pulses for effective air removal.
Conceptual design and modular construction with exclusive optional kits.
Set of four castors for easy moving and installation in lab room.
Easy to configure, 14 free configurable automatic programs.
Multilevel passwords protected key-users access to ensure process safety.
For remote monitoring via Ethernet protocol.
Special test programs for routine check of sterilizer efficiency
Technical service area:
Access by a wide front door with a special safety lock
for easy maintenance.
Full and easy accessibility to technical area from front and lateral sides.
Integrations:
Easy installation and quick connection to the utilities.
Innovative and unique modular design.
Wide selection and configurations of options on a modular system.
External trolleys fully compatible with Glassware Washer assure cleaning
integration.
Integrated software and Process controller for glassware washers steam
sterilizers, making training and maintenance easier. |
Glassware Washer:

|
The power of steam: a cost-effective cleaning:
Optimized emollient effect on greasy and sticky dirt.
Significantly reduces operating costs
True eco-friendly solution, minimize detergents, water consumption, Lowering the
running costs per cycle.
Able to access hard-to-reach areas and therefore clean thoroughly.
|
Continous monitoring:
Drain water conductivity purity set-point further reducing water and other
utilities consumption.
The cleaning process is constantly supervised.
Dedicated probes monitoring the temperature of the air/water and of the steam in
chamber.
A dedicated trasducer controls the pressure of the circulating water.
An internal LED lamp operates and signals alarm evidents by changing color
during the whole cycle. |
Design and technical features:
All compliant with cGLP standards.
Process controller, engineered and pre-validated according to GAMP5.
Rigorous sanitary finishing of piping and all equipments and CIP to ensure a
perfect washing.
Standard or customized modular racks for loads configurations and pipe
connection.
Easy and friendly ergonomic loading height, process controller.
Interchangeable external trolleys compatible with paralleled lab sterilizers.
Integrated software and Process controller for glassware washers steam
sterilizers, making training and maintenance easier.
|
Global distribution locations:
|
White Papers :
Technical Report No. 29 (Revised 2012) Points to Consider for Cleaning
Validation
https://store.pda.org/tableofcontents/tr2912_toc.pdf
Continued process verification
https://en.wikipedia.org/wiki/Continued_process_verification
Guidance on aspects of cleaning validation in
active pharmaceutical ingredient plants
http://www.chromnet.net/Taiwan/Cleaning
Strategy/APIC_Cleaning_Validation_2014.pdf |
|
|
|
|