Enzymaster R&D Collaboration Services
Enzyme
screening / Enzyme directed evolution /
Enzyme production by fermentation / Enzymatic process optimizing & up-scaling

More than
one hundred years ago, biocatalysis was employed to do chemical transformations
on non-natural man-made organic compounds, with the last 30 years seeing a
substantial increase in the application of biocatalysis to produce fine
chemicals, especially for the pharmaceutical industry.
Since almost all enzymes are made from L-amino acids, enzymes are chiral
catalysts. As a consequence, any type of chirality present in the substrate
molecule is "recognized" upon the formation of the enzyme-substrate complex,
and
both enantiomers of a racemic substrate may react at different rates.
Enzymes display
major types of selectivities:
-
Chemoselectivity
-
Regioselectivity
-
Diastereoselectivity
-
Enantioselectivity
These are the major reasons why synthetic chemists have
become interested in biocatalysis.
This interest in turn is mainly due to the
need to synthesise enantiopure compounds as chiral building blocks for
Pharmaceutical drugs and agrochemicals.
Other important advantages :
Environmentally acceptable: being completely degraded in the environment.
Minimizes side-reactions:
The enzymes act under mild conditions, which
minimizes problems of undesired side-reactions such as decomposition,
isomerization, racemization and rearrangement, which often plague traditional
methodology.
Very high stability and re-usability: Immobilized enzymes
demonstrate very high stability and re-usability and can be used to conduct
reactions in continuous mode in microreactors.
https://en.wikipedia.org/wiki/Biocatalysis
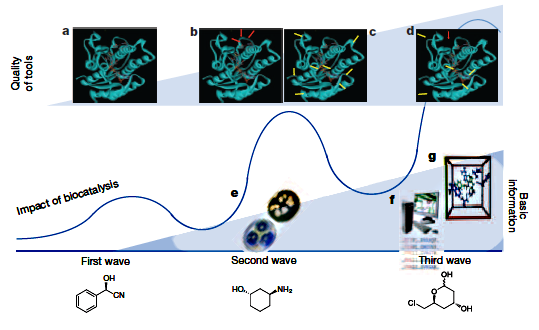
Engineering The Thirdwave of Biocatalysis
http://www.biology.uoc.gr/courses/BIO113_Enzimiki_biotexnologia/images/documents/5.pdf
https://www.ncbi.nlm.nih.gov/pubmed/22575958
The third, and present, wave of
biocatalysis started with the work of Pim Stemmer and Frances Arnold in the mid
and late 1990s. They pioneered molecular biology methods that rapidly and
extensively modify biocatalysts via an in vitro version of Darwinian evolution.
The methods are now commonly called
Directed Evolution,........
Key advances in
advanced protein engineering, gene synthesis, sequence analysis, bioinformatics
tools and computer modelling are at the base of tremendous progress in tailoring
biocatalysts by protein engineering and design, and the ability to reorganize
enzymes into new biosynthetic pathways.
As a result of the advances made during the present wave of biocatalysis,
remarkable new capabilities can now be engineered into enzymes.
Remarkable new
capabilities:
-
Ability to accept previously inert substrates.
-
Change the nature of formed product.
-
Can remain stable at 60C in
solutions containing organic solvents.
-
Can accept new substrates
-
Can catalyse new non-natural reactions.
-
Years work may now take only a few months.
Biocatalysis: Successfully Crossing Boundaries:

“ … Today, enzymes can easily be
engineered for new reactions and to match process demands …”
-
Sequence Databases Foster the
Discovery of Novel Enzymes
-
Computational Tools Facilitate
Enzyme Design
-
Combining Chemo- and Biocatalysis
-
Multistep Biocatalysis or
Metabolic Engineering?
-
New Emerging Chemistry
http://www.chemistryviews.org/details/ezine/8642801/Biocatalysis_Successfully_Crossing_Boundaries.html
http://onlinelibrary.wiley.com/doi/10.1002/anie.201510042/pdf
International Team for Substantial Service,
Supports and Cooperations :






Our team
partners, Enzymaster is a Chinese-multinational Joint company in the high-tech
hubs in China's coastal areas. Having established close collaboration with
numerous domestic companies, we specialised on providing Enzyme Catalysis
Solutions to the pharmaceutical and fine chemical industries.
Our collaborative working includes development of new Enzyme-Catalysis
technology for various high value chiral intermediates, that also facilitate the
transformation of the pharmaceutical and fine chemical manufacturing processes
towards environmentally friendly and cost effective processes.
http://www.enzymaster.com/en/index.asp
http://www.enzymaster.com/index.asp
http://www.chromnet.net/

Multinational and Multidisciplinary Team
The team members of Enzymaster have multinational
origins, such as Germany, Singapore, Malaysia, Australia and China.
We work closely as a multidisciplinary team by combining respective expertise in
various scientific aspects.

|
Dr. David Rozzell
Joined Provivi in August 2015 as Sr VP to head the pharmaceutical division of
the company. Dr. Rozzell has more than 25 years of experience in the
biotechnology industry developing and building innovative companies. He is the
inventor on 24 issued US patents and various pending patent applications. Prior
to joining Provivi Dr. Rozzell created Sustainable Chemistry Solutions, Inc. as
a consultancy and provider of information products to the enzyme industry.
Previously, he was the Founder of BioCatalytics, Inc., a pioneering developer of
enzymes and biocatalysis technology that was acquired by Codexis, Inc. in 2007.
Dr. Rozzell received a PhD in Chemistry from Harvard University and a BS in
Chemistry from the University of Virginia.
He publishes the Enzyme Industry Newsletter, along with two comprehensive
industry guides: The Enzyme Company Guide and The Biocatalysis Company Guide.
site:
http://www.bio-catalyst.com |
Prof. Dr. Christian
Wandrey
http://www.fz-juelich.de/SharedDocs/Personen/IBG/IBG-1/EN/Research_groups/general/wandrey.html?nn=547728
Former Head of Institute of Biotechnology 2 Christian Wandrey was Professor of Biotechnology at the University
of Bonn and head of the Institute of Biotechnology 2 at Forschungszentrum Jülich
from ..... Professor Wandrey's special research interest was bioprocess
development with main focuses in enzyme technology and fermentation technology.
More than 330 scientific publications, about 100 patents and patent
applications, and over 400 seminars present his scientific work. He is
co-initiator of 4 start-up companies, co-editor of 2 books, and member of the
editorial boards of various scientific journals. |

|
Leading Enzyme Directed Evolution Technology
The platform technology of Enzymaster is on par with the state-of-the-art around
the globe, with strong capability in molecular biology and high throughput
biochemistry.
Independent R&D
We have a strong focus on R&D helding ourselves to high professional
conducts,and take pride to be able to independently create high value, novel
Intellectual Property in-house.
Technology Commercialization
We are the first commercial entity in Chinese area that focuses on employment of
enzyme directed evolution technology for commercial scale production, with
reliable and capable R&D partnership for our collaborators.
Collaborative Projects
Enzyme Screening
We have a wide variety of enzyme panel, such as ketoreductases, transaminases,
hydrolases, and oxygenases. The collaborator shall provide the related material
for the screening (such as substrate, product, analytical methods, and HPLC
columns), and we shall screen for suitable starting enzyme using the optimized
analytical methods, to ascertain the enzyme activity and selectivity. Based on
the enzyme screening results, we shall evaluate the commercial viability of the
enzyme and the processes, as well as the R&D investment needed, if any, to
achieve commercial viability.
Enzyme Directed Evolution
With our core Enzyme Directed Evolution Platform technology, the properties of
selected enzyme, such as activity, selectivity, and stability, can be
improved. The evolution project will be staged into suitable phases, each with a
specific target and milestones to achieve, with the ultimate aim of achieving
commercial viability. |

|

|
Enzyme Production by Fermentation
We develop robust and highly productive fermentation processes for the
commercial production of enzyme, and then transfer the process to the
collaborator.
Enzymatic process optimizing & Up-scaling
With comprehensive consideration over the enzyme properties, we design and
develop the commercially viable enzyme-catalyzed process and downstream process
for large-scale chemical manufacturing. The technology is optimized for high
productivity and robustment at large scale. Based on the technology transfer
package, the process can be verified in the collaborator’s lab, pilot plant and
finally the production plant.
|
Directed Evolution
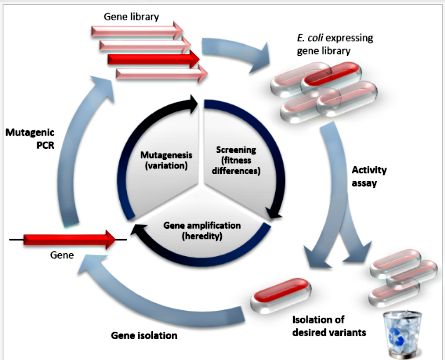
https://en.wikipedia.org/wiki/Directed_evolution
Directed evolution (DE) is a method used in protein engineering that mimics the
process of natural selection to evolve proteins or nucleic acids toward a
user-defined goal.[1] It consists of subjecting a gene to iterative rounds of
mutagenesis (creating a library of variants), selection (expressing the variants
and isolating members with the desired function), and amplification (generating
a template for the next round). It can be performed in vivo (in living cells),
or in vitro (free in solution or microdroplet).
Directed evolution is used both for protein engineering as an alternative to
rationally designing modified proteins, as well as studies of
fundamental evolutionary principles in a controlled, laboratory environment.
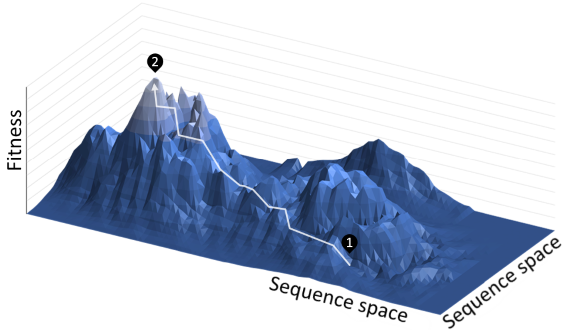
Principles:

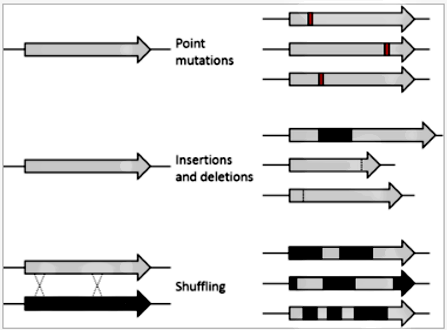
Directed evolution is analogous to climbing a hill on a 'fitness landscape'
where elevation represents the desired property. Each round of selection samples
mutants on all sides of the starting template (1) and selects the mutant with
the highest elevation, thereby climbing the hill. This is repeated until a local
summit is reached (2).
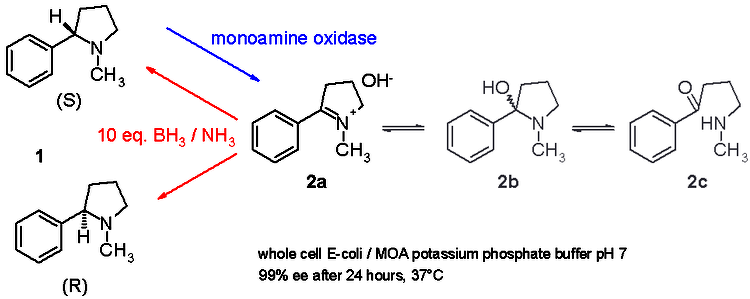
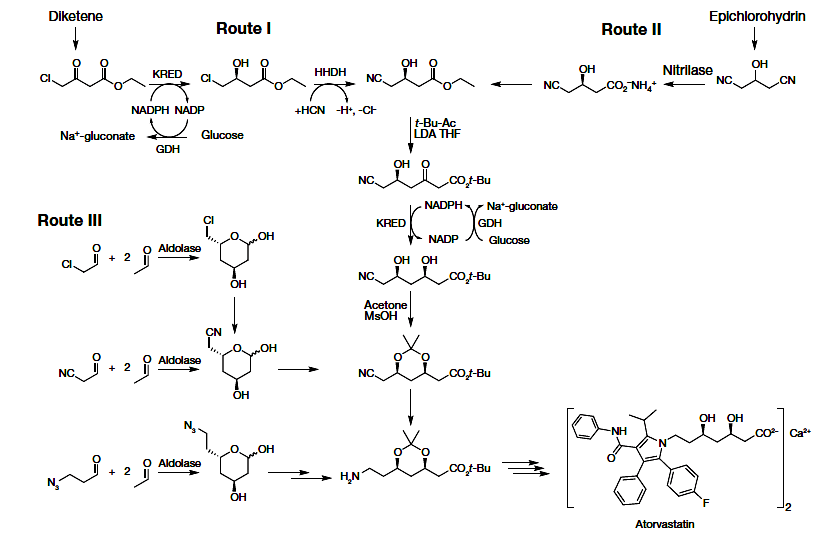
Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to
Sitagliptin Manufacture
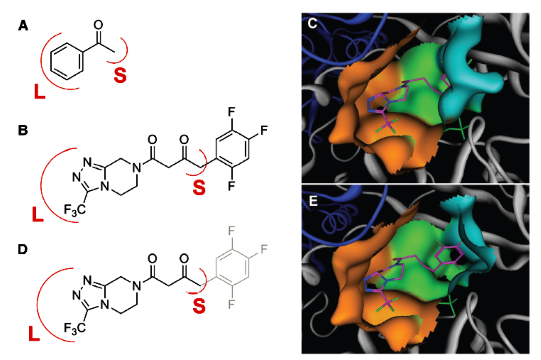
https://www.ncbi.nlm.nih.gov/pubmed/20558668
Pharmaceutical synthesis can benefit greatly from the selectivity gains
associated with enzymatic catalysis
Here, we report an efficient biocatalytic process to replace a recently
implemented rhodium-catalyzed asymmetric enamine hydrogenation for the
large-scale manufacture of the antidiabetic compound sitagliptin. Starting from
an enzyme that had the catalytic machinery to perform the desired chemistry but
lacked any activity toward the prositagliptin ketone, we applied a substrate
walking, modeling, and mutation approach to create a transaminase with marginal
activity for the synthesis of the chiral amine; this variant was then further
engineered via directed evolution for practical application in a manufacturing
setting. The resultant biocatalysts showed broad applicability toward the
synthesis of chiral amines that previously were accessible only via resolution.
This work underscores the maturation of biocatalysis to enable efficient,
economical, and environmentally benign processes for the manufacture of
pharmaceuticals.
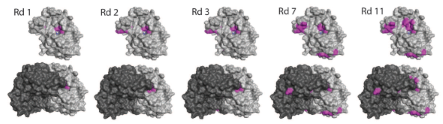
Computational Design of an Enzyme Catalyst for a Stereoselective Bimolecular
Diels-Alder Reaction
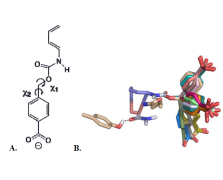
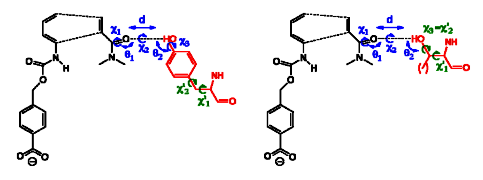
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3241958/
http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.169.2720&rep=rep1&type=pdf
The Diels-Alder reaction is a cornerstone in
organic synthesis, forming two carbon-carbon bonds and up to four new
stereogenic centers in one step. No naturally occurring enzymes have been shown
to catalyze bimolecular Diels-Alder reactions. We describe the de novo
computational design and experimental characterization of enzymes catalyzing a
bimolecular Diels-Alder reaction with high stereoselectivity and substrate
specificity. X-ray crystallography confirms that the structure matches the
design for the most active of the enzymes, and binding site substitutions
reprogram the substrate specificity. Designed stereoselective catalysts for
carbon-carbon bond-forming reactions should be broadly useful in synthetic
chemistry......
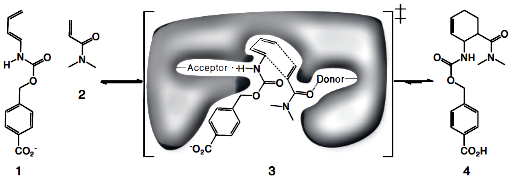
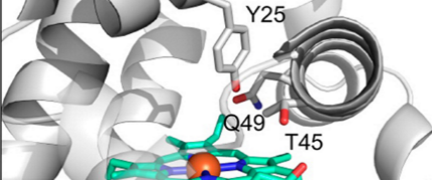
The Diels-Alder reaction. Diene (1) and dienophile (2) undergo a pericyclic [4 +
2] cycloaddition (3) to form a chiral cyclohexene ring (4). Also shown in (3) is
a schematic of the design target active site, with hydrogen bond acceptor and
donor groups activating the diene and dienophile and a complementary binding
pocket holding the two substrates in an orientation optimal for catalysis.....
|
|