|
A protease (also called a peptidase or proteinase) is any enzyme that performs
proteolysis,
that is, begins protein catabolism by hydrolysis of the peptide bonds that link
amino acids together in a polypeptide chain.
Proteases can be found in animals, plants, bacteria, archaea and viruses.
Proteases have evolved multiple times, and different classes of protease can
perform the same reaction by completely different catalytic mechanisms.
The field of protease research is enormous. in 2004, approximately 8000 papers
related to this field were published each year.
Proteases are used in industry, medicine and as a basic biological research
tool.
Digestive proteases are part of many laundry detergents and are also used
extensively in the bread industry in bread improver.
A variety of proteases are used medically both for their native function (e.g.
controlling blood clotting)
or for completely artificial functions (e.g. for the targeted degradation of
pathogenic proteins).
Highly specific proteases are commonly used to cleave fusion proteins and
affinity tags in a controlled fashion.
Guideline for Industry:
Quality of Biotechnological Products: Analysis of the Expression Construct in
Cells Used for Production of r-DNA Derived Protein Products
Points to Consider in the Production and Testing of New Drugs and Biologicals
Produced by Recombinant DNA Technology
Recommendations for the Early Food Safety Evaluation of New Non-Pesticidal
Proteins Produced by New Plant Varieties Intended for Food Use
Quality Considerations in Demonstrating Biosimilarity of a Therapeutic Protein
Product to a Reference Product
Industrial Applications of a New Biochemical
Leading with Quality, Performance and Cost :
Our partners, the Yaxin Biotechnology is a high-tech enterprise that focuses on
researching and producing the recombinant enzymes.
http://www.yaxinbio.com/en/
YaxinBio is the first and only a professional company on researching and
producing the recombinant carboxypeptidase B and recombinant trypsin in China.
Both of the two recombinant enzymes are specially used in the recombinant human
insulin production, and exporting abroad.
Recombinant trypsin is used in the cell culturing process, which conforms to the
2014 USP.
Animal original free is very important for the production of antibody and
vaccine .
Usage of recombinant trypsin fundamentally solves the problem of animal original
virus contamination in the recombinant human insulin production.
Insisting on “scientific and technological innovation”,
our team
have focused on researching and manufacturing
the animal free Biological pharmaceutical raw materials,
and have developed high
performance recombinant
enzymes and sequencing grade enzymes with global quality,
independent intellectual property rights and much helpful costs.
Products List:
Product
Information |
Product Number |
Performance |
Packing Specifications |
Applications |
|
Sequencing Grade Carboxypeptidase B |
SRCPB01 |
NLT 200 unit/mg pro |
100μg;1mg |
protein structure and sequence analysis, antibody quality control. |
|
Recombinant carboxypeptidase B |
RCPB01170 |
NLT 170 unit/mg pro |
10mg;100mg;1g |
Insulin and antibody production |
|
Sequencing Grade Modified Recombinant Trypsin |
SRT0202 |
NLT 4500 USP unit/mg pro |
100μg;1mg |
Peptide mapping Peptide mass fingerprinting MS/MS spectral matching |
|
Recombinant Trypsin |
RPT0201 |
NLT 3800 USP unit/mg pro |
10mg;100mg;1g |
Cell culture, Insulin production, Animal cells culture example |
|
Recombinant Trypsin(Human) |
RHT03 |
NLT 2500 USP unit/mg pro |
10mg;100mg;1g |
Cell culture, Insulin production, Animal cells culture example |
|
Reecombinant Chymotrypsin |
RCT10 |
NLT 1000 unit/mg pro |
10mg;100mg;1g |
Peptide mapping, fingerprinting and sequence analysis |
|
Sequencing Grade Recombinant Chymotrypsin |
SRCT03 |
NLT 1500 unit/mg pro |
100μg;1mg |
Peptide mapping Protein identification MS/MS spectral matching |
|
Recombinant
Protein A |
RSPA05 |
E275/250 ≥1.2, E0.1% at 275nm=0.17-0.23 |
10mg;1g |
Antibody, Detection kit as ELISA, and Affinity gel productions. Research |
|
Recombinant
Enterokinase |
REK08 |
5U/μl |
100U;500U;1KU; 10KU;20KU;100KU |
Recombinant protein production, R & D |
|
Trypsin solution |
RTS04 |
2000BAEE unit/ml |
100ml;500ml |
Cell culture, Insulin production, Animal cells culture example |
|
Recombinant
Aprotinin |
RTI16 |
NLT 3.0EPU/mg pro |
10mg;100mg |
Cell culture, Vaccine production |
|
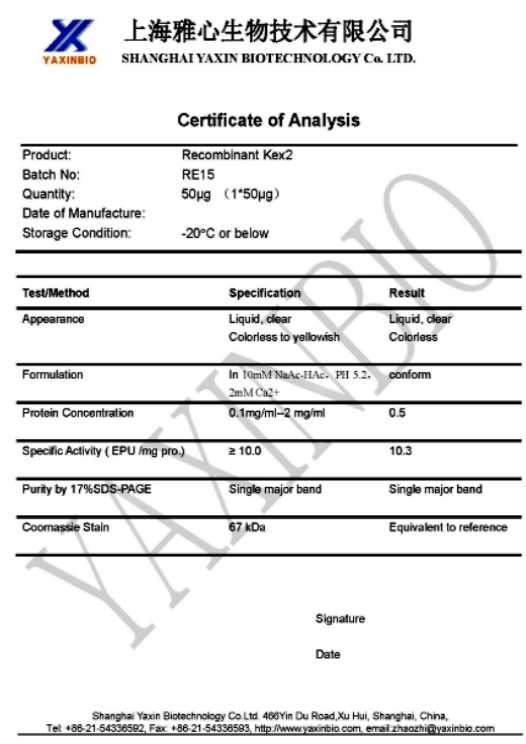
Recombinant Kex2 Protease |
Re15 |
≥10u/mg |
50µg/each |
|
|
|
Kex2 is a Ca2+-dependent serine protease and cleaves at C-terminal site of
Lys-Arg, Arg-Arg, Pro-Arg in pro-α-factor and killer-toxin precursors maturing. |
|
V8(Endoproteinase Glu-C) |
V813 |
20U/mg |
50μg,2mg |
Protein structure and sequence analysis |
|
Recombinant Proteinase K |
RPK09 |
≥30 u/mg |
1g,100g,or bulk |
A serine protease that displays the ability to digest native proteins |
|
More for inquiry...... |
|
|
|
|
Certificate of Registration / Honor / Licence / COA example:
ISO 9001:2008, Scope of Registration:
Development and Manufacture of Recombinant Protein |
High-tech Enterprise Certificate
|

|
|
|
Business License |
COA example |

|
|
International Business and Service:
As the Global Sales
agency partners and Taiwan Branch(Yaxinbio_ABDC),
we take forward the international business, that include:
1.Direct marketing
and services.
2.Services to dealers in the countries with
counseling and assistance.
3.Integration of international technical
cooperation projects.
We sincerely look
forward to your associated enforcement,
for the establishment of more helpful product services and cooperations.

Papers, Application Guides and Application
notes:
Products Descriptions:
Sequencing Grade Carboxypeptidase B

https://en.wikipedia.org/wiki/Carboxypeptidase
Catalog Number:SRCPB01
CAS: 9025-24-5
EC: 3.4.17.2
Source: Rat carboxypeptidase B, expressed in E. Coli.
DESCRIPTION
Carboxypeptidase B catalyzes hydrolysis of the basic amino acids lysine, arginine and histidine from the C-terminal end of polypeptides.
The molecular weight is 34,500 daltons, the pH optimum is 8.0, and pI is 6.0.
Carboxypeptidase B is competitively inhibited by arginine and lysine.
The enzyme is also inhibited by metal chelating agents, e.g., EDTA.
Recombinant Carboxypeptidase B is expressed in E.Coli and purified by high pressure liquid chromatography.
There is no trace of other enzyme activity.(such as carboxypeptidase A and chymotrypsin)
No protease inhibitors such as PMSF are present in the preparation.
MAIN FEATURES
|
Source
|
E. Coli
|
|
Purity by HPLC
|
≥ 95%
|
|
Format
|
Lyophilized
|
|
Specific activity
|
≥ 200 units/mg pro
|
|
Contaminant activity
|
No chymotrypsin, carboxypeptidase A, or other proteases contaminant.
|
APPLICATION
Protein structure and sequence analysis, such as hydrolyze basic amino acids lysine, arginine and histidine from the C-terminal end of polypeptides.
Antibody quality control.
RECOMMEND USAGE
To prepare 1-10mg/ml carboxypeptidase B with sterile water or 25mM Tris-HCl pH 7.65.
The ratio to aimed protein is 1:50 to 1:1000 (w/w), the optimum pH is pH 7.0-9.0.
STORAGE INSTRUCTIONS
Recommend recombinant carboxypeptidase B lyophilized should be stored under 2-8℃
in sealed container.
It is stable within 24 months.After dissolved, it should be stored under -20℃,
It is stable within 24 months and no activity lose after 10 times repeated
freezing and thawing.
Recombinant Carboxypeptidase B


Catalog Number:RCPB01
CAS: 9025-24-5
EC: 3.4.17.2
Source: Expressed in E. Coli.
DESCRIPTION
Carboxypeptidase B catalyzes hydrolysis of the basic amino acids lysine, arginine and histidine from the C-terminal end of polypeptides. The molecular weight is 34,500 daltons, the pH optimum is 8.0, and pI is 6.0.
Carboxypeptidase B is competitively inhibited by arginine and lysine.
The enzyme is also inhibited by
metal chelating agents, e.g., EDTA.
Recombinant Carboxypeptidase B (EC 3.4.17.2) is expressed in E.Coli and purified
by high pressure liquid chromatography.
There is no trace of other enzyme (such as carboxypeptidase A and chymotrypsin)
activity.
No protease inhibitors such as PMSF are present in the preparation.
ADVANTAGES
Animal origin free:YaxinBio recombinant carboxypeptidase B belongs to the AOF level 3, eliminate the risk of virus
presence,
or of any other potential adventitious agents found in animal-derived
carboxypeptitase B
StabilityA sterile recombinant carboxypeptidase B lyophilized eliminates the risk of contamination and decreases
he chances of activity loss in the process of transport and storage.
High purity:
1) Recombinant carboxypeptidase B provides increased specific activity and eliminates contaminating protease
activities found in extracted enzymes with lower purity level.
2) No other contaminating proteases such as chymotrypsin and carboxypeptidase A.
3) Less than 10ppm of recombinant trypsin.
MAIN FEATURES
|
Source
|
E. Coli
|
|
Purified by
|
HPLC
|
|
Format
|
White or white-like or yellowish lyophilized
|
|
Additives
|
Tris salts, NaCl salts and carbohydrates
|
|
Protein content
|
35% ~ 60%
|
|
Specific activity
|
≥170 units/mg pro.
|
|
Purity
|
Single main band on SDS-PAGE
|
|
M.W.
|
35kD
|
|
Contaminant activity
|
Less than 10 ppm of recombinant trypsin.
|
NIT DEFINITION:One Unit of carboxypeptidase B activity hydrolyzes one micromole of hippuryl-L-arginine per minute at 25℃, pH 7.65.
RECOMMEND USAGE :
To prepare 1-10mg/ml carboxypeptidase B with sterile water or 25mM& Tris-HCl
pH 7.65.
The ratio to ;aimed protein is 1:50 to 1:1000 (w/w), the optimum pH is pH7-9.
STORAGE INSTRUCTIONS
Recommend recombinant carboxypeptidase B lyophilized should be stored under 2℃-8℃
in sealed container.
It is stable within 24 months.After dissolved, it should be stored under -20℃,
It is stable within 24 months and above 90% activity remained after 10 times
repeated freezing and thawing.
Sequencing Grade Modified Recombinant Trypsin
https://en.wikipedia.org/wiki/Trypsin
Catalog Number:SRT0202
CAS: 9002-07-7
EC: 3.4.21.4
Source: Expressed in E. Coli, methylation modified.
DESCRIPTION
Trypsin specifically hydrolyzes peptide bonds at the carboxyl side of lysine and arginine residues.
Recombinant trypsin is free of any other proteases activities, and TPCK is unnecessary and not contained.
Unmodified trypsin is subject to auto-proteolysis, generating fragments that can interfere with protein sequencing or HPLC/MS peptides analysis.
YaxinBio’s sequencing grade modified trypsin is recombinant porcine trypsin modified by reductive methylation,
rendering it resistant to proteolytic digestion.
MAIN FEATURES
|
Source
|
E. Coli
|
|
Format
|
Liquid in 50mM HAc, or fluffy solid.
|
|
Protein concentration
|
0.5mg/ml in 50mM HAc
|
|
Specific activity
|
≥ 4500 USP units/mg pro
|
|
Purity(RP-HPLC)
|
≥99% by HPLC
|
|
Contaminant activity
|
Recombinant porcine trypsin.
No any other proteases activities contaminant.
|
UNIT DEFINITION:One USP unit of trypsin activity will produce a Delta A253 of 0.003 per minute in a reaction volume of 3.0ml at pH7.6 and 25℃
with BAEE as a substrate (1cm light path).
APPLICATION
Protein digests for peptide mapping applications or protein identification by peptide mass fingerprinting or MS/MS spectral matching.
It is suitable for digestion reactions in-solution or in-gel.
RECOMMEND USAGE
1. To dilute recombinant modified trypsin with 50mM HAc if needed. When used, dilute it with 50mM NH4HCO3 or pH7-8 buffers directly. 1mM CaCl2 is recommended to be contained in digestion buffer.
The ratio to aimed protein is 1:20 to 1:100 (w/w), the optimum pH is pH7-8.
2. To use this product, thaw at room temperature, mix gently before use.
3. No activity lost when freeze-thaw 5 cycles.
STORAGE INSTRUCTIONS
1. The solution should be stored under -70℃,
It is stable within 24 months.
2. Above 95% activity remained after 5 times repeated freezing and thawing.
3. A 0.05 mg/ml solution of sequencing grade modified recombinant trypsin
retained above 95% after a 3 hours incubation
at 37 ℃ in
50mM NH4HCOFor
long-term such as 20hours incubation, 1mM CaCl2 is
recommended to be contained.
Recombinant
Trypsin

Catalog Number:RPT0201
CAS: 9002-07-7
EC: 3.4.21.4
Source: Porcine
trypsin,
expressed in E.coli.
DESCRIPTION
Trypsin is a member of the serine protease family.
Trypsin cleaves peptides on the C-terminal end of lysine
and arginine amino acid residues.
The pH optimum of trypsin is pH 7 - 10. The enzyme is inhibited by serine
protease inhibitors, e.g. PMSF, and by metal chelating agents,
e.g., EDTA.
Recombinant Porcine Trypsin is a genetically engineered protein expressed in
E.coli
and purified by high pressure liquid chromatography.
There are no contaminating enzyme activities such as carboxypeptidase A and
chymotrypsin.
No protease inhibitors such as PMSF are contained in the preparation.
ADVANTAGES
Animal origin free:
The use of recombinant Porcine Trypsin eliminates the risk of virus presence,
and other potential adventitious agents found in animal
derived trypsin.
YaxinBio Recombinant Porcine Trypsin belongs to the AOF level 3.
Stablility:
A sterile recombinant trypsin lyophilized eliminates the contamination risks and
decreases the chance of activity loss
in the process of transport and storage.
High purity:
1)Recombinant porcine trypsin provides increased specific activity and
eliminates contaminating proteases activities
found in extracted enzymes.
2)No other contaminating proteases such as chymotrypsin or carboxypeptidase A.
MAIN FEATURES
|
Source
|
E. Coli
|
|
Purified by
|
HPLC
|
|
Format
|
White lyophilized
|
|
Additives
|
Carbohydrates
|
|
Protein content
|
35%
~
70%
|
|
Specific activity
|
≥3800 USP units/mg pro
|
|
Purity(RP-HPLC)
|
NLT 70% β-trysin, NMT 20% α-trypsin
|
|
Contaminant activity
|
No chymotrypsin, carboxypeptidase A, and other protease contaminant.
|
UNIT DEFINITION:One
USP unit of trypsin activity will produce a Delta A253 of 0.003 per minute
in a reaction volume of 3.0ml at pH7.6 and 25℃,
with BAEE as a substrate (1cm light path).
RECOMMEND USAGE
To prepare 1-10mg/ml recombinant trypsin with 1mM HCl.
The ratio to aimed protein is 1:50 to 1:1000(w/w), the optimum pH is pH7-10.
STORAGE INSTRUCTIONS
Recombinant trypsin lyophilized should be stored under 2℃-8℃
in sealed container. It is stable within 24 months.
After dissolved, it should be stored under -20℃.
It is stable within 24 months and above 90% activity remained after 10 times
repeated freezing and thawing.
Recombinant Trypsin (Human)


Catalog Number:RHT03
CAS: 9002-07-7
EC: 3.4.21.4
Source: human trypsin, expressed in E. Coli.
DESCRIPTION
Trypsin is a member of the serine protease family.
Trypsin cleaves peptides on the C-terminal end of lysine
and arginine amino acid residues.
The pH optimum of trypsin is pH 7 - 10.
The enzyme is inhibited by serine protease inhibitors,
e.g. PMSF, and by metal chelating agents, e.g., EDTA.
Recombinant Human Trypsin is a genetically engineered protein expressed in E.coli and purified
by high pressure liquid chromatography.
There are no contaminating enzyme activities such as carboxypeptidase A and chymotrypsin.
No protease inhibitors such as PMSF are contained in the preparation.
ADVANTAGES
Animal origin free:The use of recombinant Human Trypsin eliminates the risk of virus presence,
and of any other potential adventitious agents found in animal pancreas-derived trypsin.
YaxinBio Recombinant Human Trypsin belongs to the AOF level 3.
Recombinant human trypsin:The amino acid sequence is the same as the Human Trypsin 2.
Stable:
A sterile recombinant human trypsin lyophilized eliminates the contamination
risks
and decreases the chance of activity loss in the process of transport and
storage.
High purity:
1) Recombinant human trypsin provides increased specificity and eliminates
contaminating activities found in lower purity enzymes.
2) No other contaminating proteases such as chymotrypsin or carboxypeptidase A.
3) Purity is more than 95% by HPLC.
MAIN FEATURES
|
Source
|
E. Coli
|
|
Purified by
|
HPLC
|
|
Format
|
White or White-like lyophilized
|
|
Specific activity
|
≥ 2500 USP u/mg pro.
|
|
Purity
|
≥ 95% by HPLC
|
|
Contaminant activity
|
No chymotrypsin, carboxypeptidase A, and other protease contaminant.
|
UNIT DEFINITION:
One USP unit of trypsin activity will produce a Delta A253 of 0.003 per minute
in a reaction volume
of 3.0ml at pH7.6 and 25℃,
with BAEE as a substrate (1cm light path).
TORAGE INSTRUCTIONS
Recombinant human trypsin lyophilized should be stored under 2℃-8℃ in
sealed container.
It is stable within 24 months.
After dissolved, it should be stored under -20℃.
It is stable within 24 months and above 90% activity remained after 10 times
repeated freezing and thawing.
Recombinant Human
Chymotrypsin

https://en.wikipedia.org/wiki/Chymotrypsin
Catalog Number:RCT10
CAS: 9004-07-3
EC: 3.4.21.1
Source: human chymotrypsin, expressed in E. Coli.
DESCRIPTION
Chymotrypsin is a recombinant serine endopeptidase expressed in E.coli, purified with HPLC,
the gene sequence is the same as human chymotrypsin.
Recombinant chymotrypsin hydrolyzes at the carboxyl side of aromatic amino acids residues:
Tyr, Phe and Trp. Cleavage may also be observed, but at a lower rate, at Leu and Met.
Chymotrypsin activity is optimal in pH 7.0–9.0.
ADVANTAGES
Animal origin free:Recombinant human chymotrypsin eliminates the risk of virus presence, and other
potential adventitious agents found in animal-derived chymotrypsin.
High purity:Recombinant Chymotrypsin provides increased specificity and eliminates contaminated
other proteases activities found in enzymes purified from pancreas.
Stable:A
sterile recombinant human chymotrypsin lyophilized decreases the contamination
risks and
chance of activity loss during transport and storage.
APPLICATION
Hydrolysis of proteins by chymotrypsin alone or in combination with other proteases.
Suitable for peptide mapping, fingerprinting, and sequence analysis.
MAIN FEATURES
|
Source
|
E. Coli
|
|
M.W.
|
26,950 Da
|
|
Purified by
|
HPLC
|
|
Format
|
White lyophilized
|
|
Specific activity
|
≥1000 unit/mg pro
|
|
Purity
|
> 95% by HPLC
|
UNIT DEFINITION : One unit will hydrolyze 1.0 μmole of BTEE per min at pH 7.8 at 25 °C.
RECOMMEND USAGE
To prepare 1-10mg/ml with 1ml 50mM HAc, used within 2 days, or stored below -20℃ after repacked
STORAGE INSTRUCTIONS
Recombinant Chymotrypsin lyophilized should be stored under 2℃-8℃
in sealed container.
It is stable within 24 months. After dissolved, it should be stored under -20℃.
Sequencing Grade Recombinant Chymotrypsin

Catalog Number:SRCT03
CAS: 9004-07-3
EC: 3.4.21.1
Source: human chymotrypsin, expressed in E. Coli.
DESCRIPTION
Chymotrypsin is a recombinant serine endopeptidase expressed in E.coli, purified with HPLC,
the gene sequence is the same as human chymotrypsin.
Recombinant chymotrypsin hydrolyzes at the carboxyl side of aromatic amino acids residues:
Tyr, Phe and Trp. Cleavage may also be observed, but at a lower rate, at Leu and Met.
Chymotrypsin activity is optimal in pH 7.0-9.0.
The sequencing grade human chymotrypsin can be used alone or in combination with other
proteases to produce protein digests for peptide mapping applications or protein identification
by peptide mass fingerprinting or MS/MS spectral matching.
It is suitable for digestion reactions in-solution or in-gel.
MAIN FEATURES
|
Source
|
E. Coli
|
|
M.W.
|
26,950 Da
|
|
Purity
|
≥ 98 % by HPLC.
|
|
Format
|
Lyophilized
|
|
Specific activity
|
≥ 1500 units/mg pro
|
APPLICATION
1) Protein digests for peptide mapping applications or protein identification by
peptide mass fingerprinting
or MS/MS spectral matching. It is suitable for digestion reactions in-solution
or in-gel.
2) This sequencing grade enzyme can be used alone or in combination with other
proteases to produce.
RECOMMEND USAGE
For dissolving
buffer ,it is 50mMHAC,
to get the terminal concentration of 0.5μg/μl,
used once time or stored below -20℃.
For the digestion buffer,
in general,
it is 50mM or 100mM H4HCO3 to
dissolve the aim protein and then
added chymotrypsin (in general,
W:W=1:50) .
Self digestion may occur if temperatures above 37°C.
STORAGE INSTRUCTIONS
Recombinant Chymotrypsin lyophilized should be stored under 2℃-8℃ in
sealed container.
It is stable within 24 months.
After dissolved, it should be stored under -20℃.
It is stable within 24 months and above 90% activity remained after 10 times
repeated freezing and thawing.
Recombinant Protein A

https://en.wikipedia.org/wiki/Protein_A
Catalog Number:RSPA05
CAS: 91932-65-9
Source:
Protein A mutant of Staphylococcus aureus , expressed in E. Coli.
APPLICATION:
The recombinant Protein A is a genetically engineering protein containing
IgG-binding domains.
Recombinant Protein A is ideal for purification of polyclonal or monoclonal IgG
antibodies.
Protein A binds to most human and mouse IgG subclasses (e.g., human IgG1, IgG2,
IgG4;
mouse IgG2, IgG2a, IgG2b,IgG3). It also binds to cow, guinea pig, hamster,
house,
pig and rabbit total IgG form.
Recombinant protein A can be coupled to solid separation medium (such as
agarose) for monoclonal
and polyclonal antibody purification.
Recombinant protein A can be coupled to a variety of molecules (such as
fluorescent molecules,
enzyme markers, biotin, colloidal gold and radioactive markers).
These coupled derivatives can be used in antibody test in the process of
Western-blot, ELISA or immunohistochemical tests.
PRODUCT SPECIFICATION
|
Test/Method
|
Specification
|
|
Bioburden
|
No organisms detected.
|
|
12% SDS-PAGE
|
Single major band
|
|
Coomassie Stain
|
25kD
|
|
UV Spectrum
|
E275/250 ≥1.2, E0.1% at 275nm=0.17-0.23
|
|
UV spectrometry
|
No DNA or RNA detected
|
|
Triton Content
|
No Triton contaminant.
|
|
Format
|
Lyophilized
|
|
pH stable range
|
pH 1-13
|
STORAGE INSTRUCTIONS
Recombinant Protein A lyophilized should be stored under 2-8℃in
sealed container.
It is stable within 24 months.
USAGE:
For Research or Manufacturing Purpose Only. Not for Human.
Recombinant
Enterokinase(Enteropeptidase)
https://en.wikipedia.org/wiki/Enteropeptidase
Catalog Number: REK08
CAS: 9017-74-8
EC: 3.4.21.9
Source: bovine enterokinase, expressed in E. Coli
DESCRIPTION
YaxinBio Enterokinase is a kind of highly purified recombinant bovine enterokinase.
The enzyme has been extensively purified and there are no traces of other contaminating
proteases.
Enterokinase specifically hydrolyzes peptide bond at the carboxyl side of lysine residue preceded by four aspartic acids: Asp-Asp-Asp-Asp-Lys (DDDDK).
So, Enterokinase can remove N-terminal fusion protein or tags
to get aim protein with native amino acids sequence.
ADVANTAGES
1) Protease that cleaves specifically after a lysine preceded by four aspartic acids: Asp-Asp-Asp-Asp-Lys (DDDDK)
2) No any other contaminated proteases, no non-specific cutting sites.
Recommend Usage Condition:
Cutting condition: given an example: 25mM Tris-HCl 8.0
Fusion protein concentration: 0.1-1mg/ml (total protein content: 0.5-1.0mg)
EK content: 1-2U
Temperature: 25℃
Time:
over
night or 12h-16h for digestion.
Common components influence the action of enterokinase
>200mM
imidazole or
>200mM
NaCl or 5%glycerin, the reaction may be effected.
The following suggestions are given:
1)
To receive the optimum result, please dialyze the sample to 25 mMTris-HCl, pH
8.0.
2)
If the dialysis is inconvenient, please dilute the sample to
<100mM
imidazole,
<50mMNaCl,
<5%
glycerin, and the proportion of fusion protein and EK may not be changed
(1U:0.5mg fusion protein).
3)
If there are one or more components in samples, and cannot be removed,
suggest to increase the content of EK in reaction system or extend the reaction
time.
MAIN FEATURES
|
Source
|
E.Coli
|
|
M.W.
|
25,850 Da
|
|
Specific Activity
|
One unit is defined as the amount of enzyme needed to cleave 0.5mg of fusion
protein in 12 to16 hours to get 95% completion at 25°C in 25mMTris-HCl,pH 8.0.
Substrate: a special fusion protein.
|
|
Storage
|
-20°C or below.
|
|
Stability
|
Keep cool with blue ice during shipping. Remained stable at 25°C for
one week without activity lost. No activity lost after 5 cycles of
frozen-thawing.
|
PRODUCT INFORMATION
|
Product
|
Cat.No
|
Activity
|
Packaging
|
Manufacturer
|
|
Recombinant Enterokinase
|
REK08
|
≥5 U/μl
|
100U;500U;1KU;
10KU;20KU;100KU
|
YaxinBio
|
Trypsin Solution


DESCRIPTION
Recombinant trypsin solution, is an animal component free trypsin solution
optimized for cell dissociation. It is formulated with recombinant human trypsin
expressed in E.Coli and refolded and purified by chromatography.
The sequence of recombinant human trypsin is same as human trypsin 2 sequence.
FEATURES AND BENEFITS
Recombinant trypsin, Animal component-free, eliminate the risk of virus and
mycoplasma contamination
Recombinant trypsin, no bacteria, mycoplasma, mold and virus was found and
bio-burden is conform to biological standard, eliminates the risk of viruses, or
other potential adventitious agents.
Recombinant trypsin
Recombinant trypsin owns a similar kinetic of cell detachment to native trypsin.
Soybean trypsin inhibitors and other inhibitors work the same way with
recombinant human trypsin as they do with native trypsin.
High purity
Recombinant trypsin provides high specific activity and eliminates contaminated
material found
in 1:250 trypsin or other animal origin trypsin.
High safety
The trypsin solution, ready to-use, eliminates the risk of activity loss in the
process of transport
and storage. It also eliminates the contamination risk that a lyophilized powder
has.
Convenient
Recombinant trypsin solution is formulated at an optimal concentration to
dissociate adherent cells.
Good work without EDTA
No EDTA contained in buffer. And a good cell dissociation result can be
obtained,
and then eliminate EDTA influence on cell apoptosis experimental analysis.
METHOD OF USE
Step 1. Pour 1 ml buffer solution into EP tube containing trypsin powder.
Step 2. Dissolve the trypsin powder, and then pour the solution into the vial
containing buffer solution
and mix. Best use it within 4 hours.
NOTES:
Note 1. Dissociate adherent cells under room temperature ; 37
℃ is not recommended.
Note 2. If necessary, after dissolving, immediately dispense into suitable
containers,
then stored under -20℃. To use
it, dissolve it at room temperature.
Note 3. The content of trypsin is 2000 BAEE unit/ml as above prepared.
If necessary, dilute it to 1000 unit/ml or others based on your experimental
results.
PRODUCTS INFORMATION
|
Product
|
Cat. No.
|
Activity
|
Packaging
|
Manufacturer
|
|
Recombinant Trypsin Solution
|
RTS04
|
2000 BAEE u/ml
|
100 ml,500ml
|
YaxinBio
|
UNIT DEFINITION:One
BAEE unit of trypsin activity will produce a Delta A253 of 0.001 per minute
in a reaction volume of 3.0ml at pH7.6 and 25℃ (1cm light path)
Recombinant
Aprotinin

https://en.wikipedia.org/wiki/Aprotinin
Catalog Number: RTI16
Anonym: Trypsin Inhibitor
CAS: 9087-70-1
Source: Expressed in E. Coli
DESCRIPTION
Aprotinin is a competitive serine protease inhibitor that inhibits trypsin, chymotrypsin, kallikrein and plasmin.
Aprotinin forms stable complexes with and blocks the active sites of enzymes.
Binding is reversible with most aprotinin,protease complexes and dissociating at pH >10 or <3.
Effective concentration is equimolar with protease.
RECOMBINANT, ANIMAL COMPONENTS FREE
Recombinant aprotinin is expressed in E. Coli, and purified with HPLC.
It contains no animal-derived components. This is a recombinant form of bovine lung aprotinin,
which is traditionally isolated from bovine lung by methods involving fractional precipitation, gel filtration,
and ion exchange chromatography.
MAIN FEATURES
|
Source
|
E. Coli
|
|
Purified by
|
HPLC
|
|
Format
|
Liquid
|
|
Specific activity
|
≥ 3.0 EPU/mg pro.
|
|
Purity
|
≥ 95%
|
|
Contaminant activity
|
No any other protease contaminant.
|
UNIT DEFINITION:
One trypsin inhibitor unit (EPU) will decrease the activity of 2 trypsin units
by 50%
where one trypsin unit will hydrolyze 1.0 μmole of N-benzoyl-L-arginineethyl
ether(BAEE) per sec at pH 7.6 at 25 °C.
A conversion factor for Aprotinin is: 1 EPU = 1 USP Aprotinin Unit = 1800 KIU.
STORAGE INSTRUCTIONS
Recombinant aprotinin should be stored under -20°C or below in sealed container.
It is stable within 24 months.
Recombinant Kex2
Protease

https://en.wikipedia.org/wiki/Kexin
Catalog Number:Re15
EC: 3.4.21.61
Source:Saccharomyces cerevisiae Kex2, expressed in Pichia pastoris
DESCRIPTION
Kex2 is a Ca2+-dependent serine protease and cleaves at C-terminal site of
Lys-Arg, Arg-Arg,
Pro-Arg in pro-α,factor and killer-toxin precursors maturing, it was discovered
in Saccharomyces cerevisiae.
But Kex2 can’t recognize and cut a single basic amino acid,such as carboxyl end
peptide bond of arginine and lysine.
Recombinant Kex2 is a genetically engineered protein expressed in Pichia
pastoris and purified by high pressure liquid chromatography.
The optimal pH of Kex2 protease is 9.0, and the optimal temperature is 37
℃.
It is stable in buffer (pH 5.0-6.0).
The activity of Kex2 is not affected by the conventional serine protease
inhibitors such as PMSF, TPCK, TLCK inhibition.
MAIN FEATURES
|
Source
|
Pichia pastoris
|
|
Purified by
|
HPLC
|
|
Format
|
Liquid in10mM NaAc-HAc(PH 5.2) and 2mM Ca2+
|
|
Specific activity
|
≥10u/mg
|
|
12%SDS-PAGE
|
Single main stripe
|
|
Mol.weight
|
67 kD
|
UNIT DEFINITION:
One USP unit of Kex2 activity will catalyze 1μmol Boc-QRR-pNA per minute
in a reaction volume of 3.0ml at pH8.0 and 25℃.
STORAGE INSTRUCTIONS
Recommended storage temperature: -20 °C or below.
It is stable after 5 cycles freezing and thawing.
Transport temperature: ≤ 8 °C.
It should be stored in 10mM NaAc-HAc(PH 5.2) and 2mM Ca2+.
PRODUCT INFORMATION
|
Product
|
Specific activity
|
Packaging
|
Manufacturer
|
|
Recombinant Kex2 Protease
|
≥10u/mg
|
50µg/each
|
YaxinBio
|
V8(Endoproteinase Glu-C)
https://en.wikipedia.org/wiki/Glutamyl_endopeptidase
Catalog Number:V813
CAS:66676-43-5
EC:3.4.21.19
Full Name:Endoproteinase Glu-C,from Staphylococcus aureus strain V8,V8 Protease
DESCRIPTION
Staphylococcus aureus Protease
V8 (Endoproteinase GluC) is a serine proteinase that selectively cleaves peptide
bonds C-terminal
to glutamic acid residues. It also cleaves at aspartic acid residues.
The Optimum pH is 8.0 ~ 8.5. Its enzyme inhibitors are Phosphoric acid
diisopropyl ester fluoride (DFP)
and alpha 2 - macroglobulin and Na-p-tosyl-L-lysine chloromethyl ketone (TLCK).
SPECIFIC ACTIITY
Approx.20U/mg ar 25℃
with Z-Phe-Leu-Glu-4-nitranilide as the substrate(approx.500U/mg at 37℃
with casein as the substrate).
RECOMMEND USAGE
Cutting condition:PH 8.0~8.5
PACKAGING
1U/vial,50µg/vial
STORAGE INSTRUCTIONS
V8 Protease should be stored
under -20℃
in sealed container.
APPLICATION
Protein structure and sequence
analysis
Recombinant proteinase K
https://en.wikipedia.org/wiki/Proteinase_K
INTRODUCTION
Proteinase K is a non-specific endonuclease protein, belongs to the serine
protease enzyme, can cut the ester and peptide bond on the carboxy-terminal of
aliphatic, aromatic and hydrophobic amino acids, and is used for protein
degradation in biological samples.
The Molecular weight is 28.9 kDa (monomer), is active under the conditions of
pH4 to pH12 , is also very stable while the presence of SDS, urea, or EDTA.
This enzyme was purified by discoloration and chromatography to remove RNA and
DNA, without other miscellaneous activity be detected.
Since the stability of proteinase K in urea and SDS, and also has the ability to
degrade the natural proteins, it hydrolyses four peptide molecules at minimum.
PRODUCT BENEFITS
High purity: No other miscellaneous activity: Enzyme cutting with no other side
effects. High yield.
High specific activity: specific activity of not less than 30u/mg protein
Electrophoresis purity: SDS-PAGE electrophoresis, more than 99%
Stability: lyophilized powder, easy to store and transport
USAGE
Mainly used in genetic diagnostic kits, genomic DNA extraction kit, RNA
extraction kit for removing the nuclease in the preparation of DNA and RNA.
Also used for degradation of protein-containing impurities in the extraction of nonprotein component in tissue,
such as in the preparation of DNA
vaccines and heparin , etc., may also be used for the preparation of chromosomal
DNA and protein blotting.
PRODUCT FEATURES
|
Source
|
Tritirachium album limber
|
|
Molecular
weight
|
Theoretical MW:
28.9 kDA
|
|
Activity
|
activity Unit Definition: under 37
℃, pH 7.5
conditions, proteinase K amount to generate 1 μmol tyrosine per minute by from
the hydrolysis of the substrate casein is defined as one unit (U). |
|
Storage
|
can be
cryopreserved at 0 ~ 4
℃ condition for dry
powder status.
With -20
℃
storage for dissolved aliquot of appropriate volume.
|
|
Stability
|
transportation
can be carried out at room temperature.
|
PRODUCT INFORMATION
|
Product Name
|
Catalog Code
|
Activity
|
Packaging
|
Origin
|
|
Recombinant proteinase K
|
RPK09
|
≥30 u/mg
|
1g,100g,or bulk
|
YaxinBio
|
More custom items for inquiry......
|